The integrated pest management strategic plan process
This strategic plan identifies pest management priorities and integrated pest management opportunities for Oregon's nursery industry. Growers, commodity-group representatives, pest control advisors, university specialists and other ornamental nursery experts created this plan. The group met virtually in July 2021 to review details about the state's nursery production practices, challenges, success and concerns and identify critical IPM needs. The resulting plan identifies major pests, existing IPM practices and research, and educational and regulatory needs.
Contents
- 2021 IPMSP for ornamental nursery working group members
- IPM critical needs by category
- Oregon's nursery industry: An overview
- Oregon Department of Agriculture Nursery Program
- Nursery and Christmas Tree Program and nursery working group
- Interregional Research Project No. 4 Program
- IPM strategies in nursery production
- Emerging pests
- Resources and references
- Appendix A. Using PAMS terminology
- Appendix B. Pest profiles
Members of this group provided information about nursery production processes, challenges, successes and concerns. This information formed the basis of discussion for a virtual meeting on July 28, 2021. The group reviewed details for accuracy and identified IPM critical needs. This plan outlines major pests, current integrated management practices and critical needs for research, education and regulation of Oregon’s ornamental nursery industry.
Critical IPM needs emerged from work group interviews and surveys.
This strategic plan includes:
- An overview of the state nursery industry.
- Brief descriptions of pest biology.
- An alphabetical list of destructive insects, invertebrates, pathogens and weeds.
- Management practices and challenges for each pest type.
- Whole-season management practices and constraints.
- A "Prevention Avoidance, Monitoring and Suppression" framework for management practices that identifies needed tools or practices. (See "Appendix A. Using PAMS terminology.")
IPM critical needs by category
In surveys and comments, work group members identified the following urgent education, research and regulation needs:
Education
Top priority
- Beneficial insects.
- The relationship between clean plants, scouting, sprays and beneficials.
- Best management or IPM practices for new pests and diseases (Japanese beetle, spotted lanternfly, boxwood blight) to reduce disruption of IPM management plans.
Medium priority
- The effect of pollen on insect populations. (For example, high populations of thrips aren't damaging plants; they are eating pollen.)
- Good bugs and bad bugs: Presence doesn't equal problem.
- Running leafrollers and leaftier models (for ornamental plants as compared to fruit trees)
- Best management or IPM practices for new pests and diseases (Japanese beetle, spotted lanternfly) to reduce disruption of IPM management plans.
- Fire blight research and management.
Low priority
- Thrips life cycle educational materials.
- Fusarium identification tools and guides.
- Horsetail control educational materials.
Additional suggestions (provided in comments in ranking survey)
- Spanish-language materials.
- Consolidation of educational resources and making past presentations available (such as those presented at the annual Oktoberpest event at North Willamette Research and Extension Center).
- Educational materials covering resistance issues for insects, weeds and diseases combined.
Regulation
Not sorted by priority
- Clarify label restrictions (for example, when a target pest is on the label but not on the usage site, or vice versa).
- Make it easy to find rules, restrictions and guidelines, or collect industry rules in a central place.
- Clarify rules on shipping and quarantine.
- Better publicize summaries on regulations and shipping requirements for public and industry.
Research — insects
High priority
- IPM strategies for thrips management (Figure 1).
Medium priority
- Root weevil management (Figure 2).
- Thrips (specifically biological control solutions).
- Mites (two-spotted, broad, others).
- Pod gall midge — product efficacy and alternatives.
- Aphid management (all species).
- Development of thresholds for ornamental production systems.
- Aphid species and their associated viruses.
Low priority
- Flatheaded borer control (general).
- Cucumber beetle management.
- Colorado potato beetle management.
- Flea beetle management.
- Lygus bug management.
- Wireworm (as a pest of drip tape).
- Aphid taxonomy and life history.
- Symphylan control.
- Lygus bug: relationship to disease and other pests.
- Psyllid management.
- Weevil management (specifically biological control).
- Cucumber beetle: use of cucurbitacin pheromone.
Research — pathogens and diseases
High priority
- Bacterial blight management.
- Boxwood blight management (Figure 3).
- Management of Agrobacterium.
Medium priority
- New options to manage Pseudomonas to avoid resistance.
- Differences in fire blight strains based on region.
- Differences in how Phytophthora species cause damage to plants.
- Determine how communities of multiple Phytophthora species reduce plant health.
Low priority
- New options to manage Fusarium to avoid resistance.
Research — general
High priority
- Development of IPM tactics for new or invasive pests and pathogens (for example, Japanese beetle, spotted lanternfly).
- Effect of climate change on pest and pathogen pressure in the western U.S.
- More and better models to help growers make decisions about spray timing.
Medium priority
- Development of degree-day models.
- Decision aid tools based on history and thresholds. (If populations hit a threshold, the tool provides suggested actions.)
- Development of new chemistries.
- Evaluation of new chemistries.
Research — weeds
High priority
- Nostoc management in gravel yards.
- Herbicide resistance detection and management.
- Phytotoxicity of pre- and post-emergence herbicides on diverse ornamental crops, with emphasis on new and expanding crop categories of commercial importance in Oregon.
- Development of new weed control methods including nonchemical weed control.
Medium priority
- Wild carrot control in field nursery.
- Weed control options for field-grown cut flowers.
Low priority
- Application technology to improve product coverage and uniformity while mitigating drift.
Oregon’s nursery industry: An overview
An ornamental nursery (and references to “nursery” in this document) includes any operation that produces woody ornamental perennials, ornamental shade and fruit trees, and ornamental annuals and bedding plants. This includes container and field-grown plants such as bare-root or balled-and-burlapped trees and shrubs in enclosed and open production systems. This project did not consider pests of propagation material for commodity crops (such as food or fiber crops), tissue culture, garden vegetable starts, garden fruit starts, mushrooms, aquatic plants, Christmas trees or industrial hemp. Many nursery operations produce multiple types of these products. However, this project was limited to the types of production listed above. It was also restricted to the opinions of production or wholesale nurseries and not retail nurseries open to the public. We recommend that future IPMSPs for this industry narrow the focus to specific production areas, or address sectors that were not covered for this project.
“Nursery” is a broad term that encompasses many different types of production practices and plant material types primarily grown for ornamental or landscape purposes. These products include:
- Woody perennials for landscaping.
- Annuals, including bedding plants.
- Shade and fruit trees.
- Cut flowers.
- Bulbs, corms, rhizomes and tubers.
Products are often grouped by how the product is grown or sold:
- Containerized — plants grown in in pots, liners, hanging baskets or any other container with growing media to support the plant.
- In-ground production — products grow directly in the soil free of containers.
- Bare-root trees — dormant trees shipped without soil around roots.
- Balled-and-burlapped trees — trees grown in ground where the root ball is wrapped in burlap containing soil media before shipping.
Production may take place outdoors or indoors. Outdoor production may be either in-ground or containerized, depending on the product or operation methods. Indoor or protected production methods include enclosed glasshouses, greenhouses, retractable roof greenhouses (“Cravos”) and open-end “hoop houses.” Many operations are not limited to one type of product category or production method and grow multiple types of plants to use production space year-round. (For example, growers may dedicate a percentage of their area to annuals in spring and summer. After their shipping season, they may use that area for holiday annuals and later vegetable starts.)
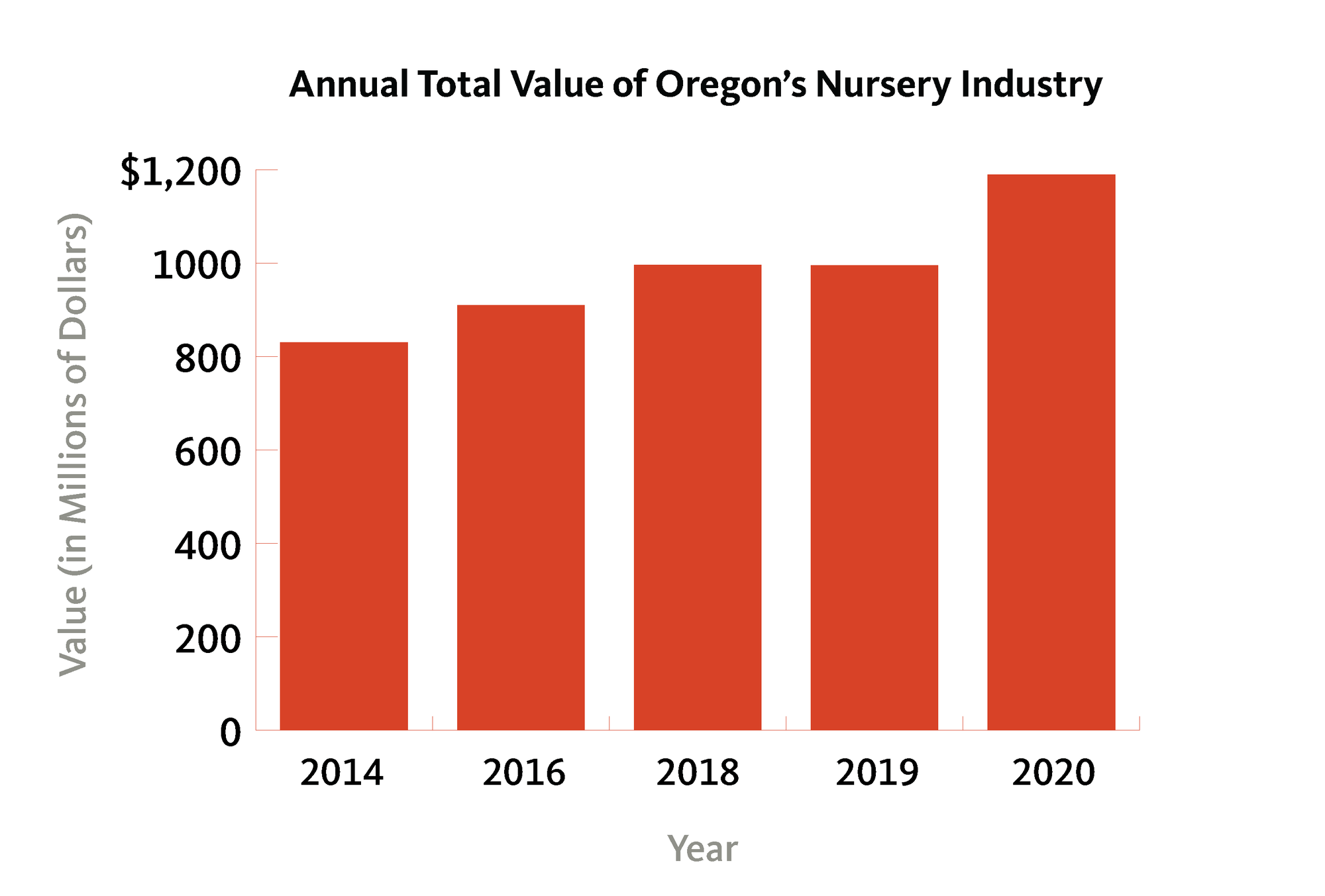
The ornamental nursery industry is one of Oregon’s top-value agricultural commodities, with total sales in 2017 reaching over $961 billion (Figure 4). In 2020, Oregon nursery products were the state's most valuable commodity, valued at nearly $1.2 billion. This figure was well above cattle and calves, the next most valuable commodity at $588 million. This was the highest value ever recorded for the nursery and greenhouse industry. Nearly all of the production occurs in the Willamette Valley and coastal growing regions of Oregon. Production nurseries can range in size from a large backyard growing a handful of native plant species to multiacre, multilocational production campuses with thousands of different plant varieties.
Table 1: Oregon nursery production at a glance
- Total employees: 14,985
- Total licensees, 2020: 2,933
- Total acres, open production: 33,854
- Total acres, greenhouse/protected: 1,437
- Total acres in production: 35,291
- Total sales, 2017: $961 million
- Total sales, 2020: $1.2 billion
Sources: NASS Census of Agriculture 2017 and 2020, Oregon Department of Agriculture and Oregon Association of Nurseries. Note: Totals do not include sectors of the nursery industry excluded from this project.
| Month | Production activities | Direct management activities |
|---|---|---|
| January | Spring propagation and planting (indoor). | Scouting for thrips, aphids, spider mites. Applications of biocontrol agents (predatory mites, spot sprays of aphids and leafhoppers while populations are low). Scout for pathogens (powdery mildew, downy mildew), treat with fungicides. |
| February | Spring propagation and planting (indoor). Spring sales begin. | Applications of biocontrol agents (predatory mites) for hanging baskets. |
| March | Spring propagation and planting. Spring sales continue. | Applications of biocontrol agents (predatory mites) for hanging baskets. Greenhouse fogging for aphids in hanging baskets (if necessary). Powdery and downy mildew treatments (fungicides). |
| April | Spring propagation and planting. Spring sales increase. | Applications of biocontrol agents (predatory mites) for hanging baskets, powdery and downy mildews, and various pathogen treatments (fungicides). |
| May | Spring propagation and planting. Spring sales peak. | Applications of biocontrol agents (predatory mites) for hanging baskets, powdery and downy mildews, and various pathogen treatments (fungicides). |
| June | Fall propagation and planting begins. Poinsettias arrive, outdoor production begins (ornamental kale and cabbages, ornamental peppers). Spring sales decline. | Applications of biocontrol agents (predatory mites) for hanging baskets, horticultural oil dips for poinsettias, biocontrol agents applied (predatory mites, rove beetles, Eretmocerus for whitefly). |
| July | Fall propagation and production (indoor and outdoor). | Applications of biocontrol agents (predatory mites) for hanging baskets, |
| August | Fall sales start. Fall propagation and production (indoor and outdoor). | Scouting for whitefly on poinsettias, manage with Eretmocerus. Sanitize open space in greenhouses and outdoor production areas. |
| September | Fall sales. Fall propagation and production (indoor and outdoor). | Scouting for whitefly on poinsettias; manage with Eretmocerus. Sanitize open space in greenhouses and outdoor production areas. |
| October | Fall sales (poinsettia, ornamental kale, peppers, garden mums). | Scouting for pests on poinsettias; manage with Eretmocerus. Sanitize open space in greenhouses and outdoor production areas. Adjustment of environmental controls for botrytis management. |
| November | Fall sales (poinsettias, primarily). | Scouting for pests on poinsettias, manage with Eretmocerus. |
| December | Sales slow. | Sanitize open space in greenhouses and outdoor production areas. |
| Year-round | - | Weed management in and around production areas, disease management through environmental control (temperature and humidity in greenhouses). |
| Month | Production activities | Direct pest-management activities |
|---|---|---|
| January | Trees for shipment in cold storage and sawdust storage | Pre-emergent herbicide applications and postemergent burn down for weeds in production areas. Scouting and sprays for disease control (mold, Botrytis). Scouting and oil sprays for mites on stored trees. |
| February | Planting begins | Pre-emergent herbicide applications and postemergent burn down for weeds in production areas. Scouting and sprays for disease control. Scouting and oil sprays for mites on stored trees. |
| March | Planting continues | Pre-emergent herbicide applications and postemergent burn down for weeds in production areas. |
| April | Planting continues | Management for anthracnose, fire blight, Pseudomonas, wooly aphid. Pre-emergent herbicide applications and postemergent burn down for weeds. |
| May | Planting continues | Management for above diseases, rust and scabs. Pre-emergent herbicide applications and postemergent burn down for weeds. Management of leafrollers and leaftiers, thrips, lygus bug, pod gall midge, symphylans. |
| June | Planting slows | Management same as May. |
| July | - | Pest management slows due to dry conditions. |
| August | - | Preventative management for Phytophthora syringae. |
| September | - | Management for Phytophthora syringae. Pre-spraying harvested trees before storage for Fusarium. |
| October | Harvest for cold storage begins | Management for Phytophthora syringae. Storage sprays for Botrytis, storage molds and Fusarium. |
| November | Cold storage management | Mold and Botrytis scouting. ODA inspections for insects. |
| December | Cold storage management | Mold and Botrytis scouting. |
| Month | Production activities | Management activities |
|---|---|---|
| January | - | Weed management: Application of pre-emergent herbicides. Insect management: Applications of dormant oils for mites and overwintering eggs. |
| February | Sales to warmer parts of U.S. | Weeds: Application of pre-emergent herbicides. Insects: Applications of dormant oils for mites and overwintering eggs. |
| March | Sales, loading, shipping. | Weeds: Application of pre-emergent herbicides. |
| April | Sales, loading, shipping. Start irrigation. | Weeds: Application of pre-emergent herbicides. Insects: Applications of dormant oils for mites and overwintering eggs, management for wooly apple aphids, drenches for lacebugs. Pathogen management: Applications of fungicides: Pseudomonas, Phomopsis, Phytophthoras. |
| May | Field fertilization. Sales, loading, shipping. | More specific pesticide applications. Weeds: Application of postemergent herbicides and spot treatment. |
| June | Sales wind down. Fertilizer applications. Cleaning of production areas. | Disease management, fungicide applications: rusts, anthracnose, powdery mildew, tip blights. Insect management: aphids, thrips. Weeds: Application of post-emergent herbicides and spot treatment. |
| July | Cleaning of production areas. | Diseases, fungicide applications: rusts, anthracnose, powdery mildew, tip blights. Insect management: aphids, thrips. Weeds: Application of post-emergent herbicides and spot treatment. |
| August | Start canning/potting for next year. Fertilize field. | Weeds: pre-emergent applications (groundsel, bittercress, willowherb). Insect management: mites, aphids, thrips. Weeds: application of post-emergent herbicides and spot treatment. |
| September | Canning/potting for next year. | Insects: root weevils, thrips. Fungicide applications for pseudomonas, blights, lead spots. Weeds: pre-emergent applications (groundsel, bittercress, willowherb). |
| October | Canning/potting for next year. | Diseases: bacterial blights |
| November | Canning/potting for next year. | Diseases: bacterial blights. Insects: dormant oils. |
| December | Canning/potting for next year. | Disease management: bacterial blights |
| Year-round | - | Biocontrol: applications of predatory mites, rove beetles in enclosed areas to suppress fungus gnats and mites. |
Oregon Department of Agriculture Nursery Program
Summary provided by Chris Benemann, program manager, Nursery and Christmas Tree, Oregon Department of Agriculture
The Oregon Department of Agriculture’s Nursery Program offers inspection and certification services to the nursery industry. The program’s goals are two-fold:
- Prevent the introduction and spread of injurious plant pests, diseases and noxious weeds within Oregon’s nursery industries.
- Assist Oregon growers in producing high-quality plant material that is competitive in domestic and international markets.
ODA officials use pest quarantines as the first line of defense to exclude exotic pests from Oregon. They also use state control area orders to slow their spread within Oregon. On average, Oregon detects nine non-native pest species per year. These are all new pests with one in five considered to be a significant economic pest, according to J. Vlach, ODA entomologist. Nursery inspectors play a critical role in acting as first detectors through the inspection of incoming plant material and checking that material is compliant with state and federal phytosanitary requirements.
The nursery program is funded almost entirely from annual license and phytosanitary certification fees. Inspection fees vary depending on the type of certification provided. Any nursery with gross sales of $250 or more is required to be licensed with the Oregon Department of Agriculture. In 2020, 2,844 nurseries were licensed with the department. The nursery program offers three types of nursery licenses: dealer, greenhouse and nursery stock growers license. The license fee for each is based on the wholesale cost of the nursery stock sold. Sellers of cut flowers do not require a license. During the 2020 calendar year, 1,644 nursery dealer licenses ($205 million reported sales); 270 greenhouse grower licenses ($137 million reported sales); and 745 nursery stock grower licenses ($758 million reported sales) were issued.
The program oversees various testing and audit-based programs, each serving a different grower audience. A few examples are:
- Phytophthora ramorum Nursery Program.
- Boxwood Blight Cleanliness Program.
- European Pine Shoot Moth Trapping Program.
- U.S.-Canadian Greenhouse Certification Program.
- U.S. Nursery Certification Program.
- Blueberry Virus Testing Program.
- Grapevine and Fruit Tree Certification programs.
- Barberry Certification Program.
Staff
The program has a staff of 12, consisting of a program manager, lead Inspector and 10 inspectors. Oregon is split into nine territories with an inspector assigned to each territory. Licensed growers within a given territory are assigned an inspector as their primary point of contact for all inspection and certification needs. The ODA maintains a list of all staff and the regions they cover.
In 2020, nursery inspectors conducted 5,713 inspections at licensed nurseries. Inspections include routine inspections, audits to fulfill compliance agreement requirements, sampling for certification testing and inspections to issue phytosanitary certificates for shipments. Inspectors issued 3,944 federal phytosanitary certificates for consignment of nursery stock (including Christmas trees) to 61 foreign countries. Canada stands as the No. 1 importer of Oregon nursery products (53%). Mexico is the second-largest importer (26%). Other countries receiving Oregon material include Australia, Azerbaijan, Brazil, Chile, Guatemala, Costa Rica, Peru, Singapore, South Africa, Switzerland, Taiwan, Thailand, the United Kingdom and Vietnam. Domestic shipments are not as common, with Oregon issuing only 756 state phytosanitary certificates in 2020.
Nursery inspectors also play the role of educator, through the dissemination of information on regulations and inspection requirements to licensed growers and the general public. They also share relevant pest and disease management information obtained through the shared relationship our program holds with entomologists, plant diagnostic labs, other state and federal agencies and academia.
ODA program partners
The nursery program works in concert with the Plant Health and Insect Pest Prevention and Management programs to achieve its goals of:
- Protecting Oregon’s agriculture, horticulture, environment and quality of life from damaging insect pests.
- Enhancing or maintaining the value of our agricultural and horticultural products.
The plant health program provides diagnostic and routine testing services for licensed nurseries. It also offers certification testing, such as that required by the ornamental and fruit tree virus certification or Phytophthora ramorum programs. In addition, they survey areas to establish pest-free status for Oregon or for specific regions or counties. This is key to ensuring market access of Oregon commodities. The Insect Pest Prevention and Management Program (known as IPPM) conducts surveys throughout the state to detect invasive species. Early detection protects our natural resources and ensures that our products meet the phytosanitary requirements of both domestic and international regions. The program also oversees and operates eradication and control programs, such as ongoing eradication of Japanese beetle in the Portland area. Like the plant health laboratory, the IPPM laboratory provides diagnostic services to the nursery industry and the general public. It also performs pest risk assessments and inspections for several state and federal permit programs.
Anyone operating as a grower or dealer of nursery stock in Oregon is required to be licensed by the Oregon Department of Agriculture. Nursery stock is defined by ODA as:
- Floral stock
- Herbaceous plants
- Bulbs
- Corms
- Roots
- Scions
- Grafts
- Cuttings
- Fruit pits
- Seeds of fruits
- Forest and ornamental trees and shrubs
- Berry plants
- Trees, shrubs, vines and plants collected in the wild that are grown or kept for propagation or sale
In order to ship products out of state, plant material must be accompanied by a shipping permit issued by the Oregon Department of Agriculture that certifies the material is regularly inspected and free of pests and diseases of concern. To be shipped out of state, nursery stock grown in Oregon must:
- Be free of pests, diseases and noxious weeds.
- Be accompanied by an ODA shipping permit.
- Meet the shipping requirements of the destination states or country.
Other states and countries also have their own restrictions about imported plant material and may require a phytosanitary certificate, which requires specific inspection by ODA or federal officials.
For more information:
OSU and USDA-ARS Nursery and Christmas Tree Program and nursery working group
Faculty and researchers across Oregon State University’s College of Agricultural Science and the College of Business work to improve and protect the state’s nursery, greenhouse and ornamental crops industries. Many of these researchers work closely with the staff of the USDA-ARS Horticultural Crops Research Laboratory in Corvallis. Research and development programs include breeding and genetics, pest management, plant health, pollination and aspects of business operations.
Find more information at the College of Agricultural Science.
Interregional Research Project No. 4 Program
The IR-4 project’s Ornamental Horticulture Program helps provide safe and effective pest management solutions for greenhouse, nursery, landscape, Christmas tree and forestry producers. IR-4 researchers work with growers, researchers, registrants and regulatory agencies to facilitate new product registrations. They also work to place new diseases, insects, weeds and crops on already registered ornamental horticulture product labels. Every other year, the group reviews the next two-year research plan and defines new priorities at its Ornamental Horticulture Workshop. Members of the industry are invited to help prioritize the research by answering questions about the diseases, insects and weeds that impact their businesses. The current program contact is Lloyd Nackley, assistant professor of nursery production, North Willamette Research and Extension Center, Oregon State University. For more information:
IPM strategies in nursery production
Integrated pest management is an approach to pest control developed in the 1960s and has been the prevailing school of thought since. IPM seeks to control pests (arthropod pests and diseases, weeds, nematodes and vertebrates) using multiple, complementary tactics in an environmentally and economically sound manner. Northwest nursery growers and advisors use IPM tools such as monitoring, sampling, scouting, predictive models, biological control, physical and cultural control, sanitation, host plant resistance, and chemical control. The goal is to keep pests below an economic threshold with a selection of these tools and to harmonize them with the control of other pests.
PAMS
PAMS — Prevention, Avoidance, Monitoring, Suppression — is a classification system for IPM developed for use by federal agencies seeking to support adoption of IPM by farmers. See Appendix A for an overview of the PAMS classification. This system provides a simple way to categorize some examples of the IPM tactics that nurseries currently use in their production systems.
Best practices for nurseries
General
Prevention —before the pest has established or appeared
- Start with clean plant material. Source plants from licensed nurseries and suppliers that arrive pest-free.
- Inspect incoming loads thoroughly for signs of pests. Reject or destroy infected material.
- Quarantine plant material coming into the nursery to observe for pests and pathogens and keep it separate from existing stock before ensuring the new material is clean.
- If possible, bring in material that is soil-, root- or leaf-free to reduce the chance of bringing in pests.
- Keep detailed records of the source of material brought into the nurseries.
- Make sure workers are not traveling between contaminated and clean areas without taking protocols to avoid transferring pests such as pathogen inoculum or weed seeds (changing gloves, coveralls, washing boots).
- Eliminate possible alternative hosts in nearby production areas. (For example, conduct weed management in adjacent fields if weed can host pathogens or pests).
Avoidance — if there are or have been occurrences of the pest
- Move infested or damaged plant material away from other material.
Monitoring — Watch out for pests or pest progression
- Regular scouting, monitoring and record-keeping of detections.
- Develop a regular record-keeping program as a decision aid tool (timing of pest occurrences, population levels, action thresholds).
- Develop an internal or custom system for management based on your products, site location, management style, etc. Some nurseries have created their own systems in part to prevent loss of institutional knowledge as staff changes. This system is integrated it into their training protocol.
- Be aware of pest alerts and invasive species alerts published by regulatory agencies or universities in both your state and nationally.
- Report incidences of suspected or unknown invasive pests or unknown pests and pathogens.
Suppression — remove the pest
From an IPM standpoint, pest suppression is often categorized using the following terms (with examples):
- Cultural — use of cover crops, mulches to outcompete or suppress weeds.
- Biological — implementation or support of natural enemies, pheromones and other biologically based methods of control .
- Chemical — applications of pesticides, including synthetic and biopesticides.
- Mechanical/physical — physical removal or destruction of pests (hand removal, flaming, mowing).
Insects
Prevention
- Use exclusion techniques to keep insects out of enclosed areas (place overlapping mesh screens, make sure doors and openings seal tightly, screen open vents).
- Manage weeds that serve as alternate hosts, especially at different times of the year.
Avoidance
- Limiting use of pesticides increases occurrences of natural enemies.
- Example: Limiting use of abamectin increased occurrences of multiple beneficial insects.
- Example: Use less frequent drenches as opposed to more frequent sprays.
- Example: Drip irrigation reduces occurrences of spider mites.
Monitoring
- Regular scouting, monitoring, and record-keeping of detections (Figure 5). Make sure that your monitoring techniques match the pests that you are trying to scout for. Use sticky cards for flying insects or indicator plants for shared pests.
- Example: Some greenhouses will grow eggplant starts and use the plants as indicators for aphids that also attack poinsettias.
- Example: monitoring and trapping for ambrosia beetles in shade tree production. Beetles are attracted using a section of tree trunk and alcohol.
Suppression
- Cultural: Reduce potential habitat near production areas if possible and within reason.
- Biological
- Deploy natural enemies in greenhouses for specific pests. Hypoapsis (a predatory mite) and Atleta (rove beetles) target fungus gnats and thrips, and Eretmocerus (a parasitic wasp) targets for whiteflies.
- Use biological predators or mycoinsecticides, with an increase in these tactics as pest pressure increases.
- Build populations of natural enemies in a production area over time.
- Chemical
- Despite a robust biological program, pesticide applications for insect pests are still frequent and necessary.
- Employ chemicals as a last resort, especially if they have a detrimental or residual effect on predators or pollinators.
- Limiting use of pesticides increases occurrences of natural enemies. Example: Use less frequent drenches as opposed to more frequent sprays. Drenches provide longer protection, and frequent sprays physically disrupt natural predators.
- Mechanical
- Physical removal of pests. Examples: hand removal of slugs and snails from pots, strong water sprays to dislodge azalea lace bugs.
Weeds
Prevention
- Use weed-free potting media and soil. Do not reuse potting media. Get assurance from suppliers that the media is free of weed seeds.
- Keep all propagation areas and instruments sterile to prevent the spread of disease. Sterilize tools after use. This includes vehicles, carts and anything used in the propagation process.
- Drip irrigation reduced weeds in center aisles.
- Mulching (for container nurseries)
- Hazelnut shells.
- Cedar sawdust is effective for winter with pre-emergents that have application restrictions (in hoop houses and closed structures).
- Rice hulls may be an option. They are working well for liverwort in greenhouse propagation.
- Solarization of seed beds to prevent weed seed germination.
Avoidance
- Pre-emergents need to be specific for the targeted species and applied at the correct time to prevent germination.
- Manage weeds before they get to reproductive stage to avoid population explosions (Figure 6). Example: When looking at priority management areas, the area with the most weeds may appear to be the priority, but it’s already infested. First, manage the areas that may have fewer weeds but are closer to reproduction. Try to keep weeds from setting seed, since the infested area is not getting any worse.
Monitoring
- Regular scouting for weeds in production areas to maintain “early detection, rapid response.”
- Record weed species presence and response to management method to inform future weed management program.
Suppression
- Mowing weeds and cover crops in alleyways.
- Flaming to suppress weeds in gravel pads.
- Mechanical or manual removal.
- Physical: solarization of seed beds for weeds and diseases to reduce broadleaf weeds where herbicides could not be used.
- Chemical: site-specific applications of post-emergents to prevent seed production based on known problem areas and known species.
- Rotate products with different modes of action to prevent resistance.
Pathogens
Prevention
- Keep all propagation areas and instruments sterile to prevent the spread of disease and sterilize tools after use. This includes vehicles, carts, clothes, boots and anything used in the propagation process.
- Sterilize or disinfest containers before reuse. Use new pots and trays when possible. Do not share pots between nurseries.
- Use pathogen-free potting media and soil. Do not reuse potting media. Get assurance from suppliers that media is pathogen-free.
- Sand, peat moss and, in some cases, bark mulch must be disinfested before use.
- Media such as perlite and vermiculite do not generally carry pathogens.
- Compost should be prepared to eliminate pathogens and viruses. Get written assurance from suppliers about these practices.
- Do not use dead and dying plants for composting media.
- Disinfect empty growing spaces as often as possible: greenhouse benches, gravel pads, any areas used for propagation or storage.
- Solarize of seed beds to kill pathogen inoculum.
- Move or grow plants on sites where pathogens of interest are absent or occur in low numbers, or grow resistant plants.
- Prevent or suppress disease by managing temperature and humidity with environmental controls in greenhouses.
- Promote air movement and circulation fans.
- Increase drainage to prevent overly wet or humid conditions or stagnant pools.
- Protection for hardy plants from frost.
- Use temperature control for root systems.
Avoidance
- Quarantine incoming shipments before you can determine that they are healthy.
- Consider watering tactics.
- Use drip tape instead of overhead irrigation to reduce leaf wetness.
- Timing, frequency and duration of irrigation can all affect disease cycles and inoculum. Consider the life conditions that will increase or decrease the chance of disease and spread of pathogens. Frequent irrigation often leads to overly wet soil and wet leaves, which may contribute to severe disease outbreaks. Example: Use of drip irrigation reduced occurrences of powdery mildew, willow blight, scab and some foliar diseases.
- Do not irrigate from infected water sources.
- Consider temperature in greenhouses.
- Manage pH, fertility and fertilizer inputs to avoid overfertilization, which may favor pathogen infection and increased disease severity.
Monitoring
- Regular scouting, monitoring and record-keeping of detections. Example: Create a list of “problem plants” that are prone to certain diseases.
- General practice: Scout, apply preventatives and use traditional fungicides if necessary (including systemic drenches) if disease pressure is high.
Suppression
- Chemical. For diseases managed through fungicides, include rotation of products with different modes of action to prevent resistance.
- Timed applications of fungicides is currently the most often used tactic for managing pathogens.
- Consider “softer” or biological chemistries first to avoid harm to beneficial insects that can lead to other pest outbreaks.
For detailed information about many of these steps, see Safe Procurement and Production Manual: A Systems Approach for the Production of Healthy Nursery Stock, published by the Oregon Association of Nurseries.
Top pests affecting nurseries in Oregon
The following were identified as current concerns, issues or challenges for the participants during interviews, surveys and the working group meeting. This does not represent an exhaustive list of all pests affecting the nursery industry. Pests are listed in alphabetical order under groupings or common names, and not ranked in importance to the industry. Following this list are tables of management notes from work group participants. See Appendix B for brief descriptions of each top pest, pathogen and virus.
Insects and invertebrates
- Ambrosia beetles, Xyloborus dispar
- Aphids (family Aphididae)
- Green peach aphid, Myzus persicae
- Cabbage aphid, Brevicoryne brassicae
- Melon aphid, cotton aphid Aphis gossypii
- Spirea aphid, citrus aphid, Aphis citricola
- Foxglove aphid, Aulacorthum solani
- Wooly aphids
- Wooly apple aphid, Eriosoma lanigerum
- Wooly ash aphid, wooly elm aphid, Eriosoma americanum
- Wooly beech aphid, Phyllaphis fagi
- Azalea lace bug, Stephanitis pyrioides
- Black vine weevil, root weevils, Otiorhynchus sulcatus
- Broad mites, Polyphagotarsonemus latus
- Cyclamen mites, Phytonemus pallidus
- Cucumber beetles
- Spotted cucumber beetles, Diabrotica undecimpunctata
- Striped cucumber beetles, Acalymma trivittata
- Cutworms and armyworms
- Beet armyworm, Spodoptera exigua
- Variegated cutworm, Peridroma saucia
- Yellowstriped armyworm, Spodoptera ornithogalli
- Flea beetles
- multiple species
- Fungus gnats, Orfelia spp. Bradysia spp.
- Honeylocust pod gall midge, Dasineura gleditschiae
- Leafhoppers (family Cicadellidae)
- Leafrollers and leaftiers (family Tortricidae)
- Lygus bug, Lygus hesperus
- Nematodes (various)
- Pacific flatheaded borer, Chrysobothris mali
- Peachtree borer, Synanthedon exitiosa
- Slugs
- Grey field slug, Deroceras reticulatum
- Multiple Arion spp. slugs including Arion hortensis and Arion rufus
- Striped greenhouse slug, Ambigolimax valentianus
- Tramp slug, Deroceras invadens
- Marsh slug, Deroceras laeve
- Greenhouse slug, Milax gagates
- Snails
- Brown garden snail, Cornu aspersum
- Amber snails (family Succineidae)
- Symphylans, Scutigerella immaculata
- Thrips (family Thripidae)
- Western flower thrips, Frankliniella occidentalis
- Echinothrips, Echinothrips americanus
- Two-spotted spider mites, Tetranychus urticae
- Whiteflies (family Aleyrodidae)
- Greenhouse whitefly, Trialeurodes vaporariorum
- Sweet potato whitefly Bemisia tabaci
Pathogens
- Fungal diseases
- Anthracnose (grouping for related fungal diseases) (Figure 7)
- Monilinia — brown rot
- Botrytis
- Fusarium
- Powdery mildew
- Bacterial blights
- Pseudomonas
- Xanthomonas
- Crown gall
- Agrobacterium
- Tip blights
- Arborvitae tip blight
- Spruce tip blight
- Phomopsis
- Leaf spots and blights
- Multiple agents
- Shothole
- Root and crown rots
- Multiple Phytophthora species
- Fusarium
- Black root rots
- Thieaviopsis
- Wilts
- Verticillium wilt
- Diseases of specific concern
- Erwinia amylovora (fire blight)
- Phytopthora ramorum (sudden oak death)
- Viruses
- Impatiens necrotic spot virus
- Tomato spotted wilt virus
- Tobacco mosaic virus
- Leaf spots and blights
Weeds
- Annual bluegrass, Poa annua (including resistant biotypes)
- Bindweed, field bindweed, Convolvulus arvensis
- Bittercress, Cardamine oligosperma and similar species
- Canada thistle, Cirsium arvense
- Common chickweed, Stellaria media
- Creeping woodsorrel, Oxalis corniculata
- Chamomile, dog fennel, Anthemis cotula
- Groundsel, Senecio vulgaris (including resistant biotypes)
- Italian ryegrass, Lolium multiflorum (including resistant biotypes)
- Lambsquarters, Chenopodium album
- Liverwort (Marchantia spp.)
- Mare’s tail, horseweed, Conyza canadensis
- Northern willow herb, Epilobium spp.
- Nostoc (cyanobacteria)
- Pearlwort, Sagina procumbens
- Pigweed, Amaranthus spp.
- Prostrate spurge, spotted spurge, Euphorbia maculata
- Quackgrass, Elytrigia repens
- Shepherd’s-purse, Capsella bursa-pastoris
- Smartweed, knotweed, Polygonum spp.
- Wild carrot, Daucus carrota
- Yellow nutsedge, Cyperus esculentus
Table 6: Current pest management strategies for key pests
The following listings are not recommendations for management. This table represents management practices that project participants say they currently use for these key pests.
Insects and invertebrates
Weeds
In addition to best management practices described earlier:
Pathogens
Diseases of specific concern
Emerging pests
Japanese beetle — Popilia japonica
Japanese beetle (Coleoptera: Scarabeidae) is a pest of landscape plants and turf in much of the eastern United States. Adults feed on foliage of over 300 different plant species. Larvae feed on the roots of plants and turf. Western states have been maintaining survey and eradication programs for the pest for over six decades to prevent its establishment. Despite eradication efforts, it has recently begun to spread in Colorado. Oregon Department of Agriculture places thousands of traps annually to detect Japanese beetle introductions into the state. Interceptions and eradication activities occur regularly each year at the Portland International Airport, as Japanese beetle are often transported with arriving cargo planes. In 2015, a population was detected in Washington County. A widespread eradication and quarantine program is underway. In spring 2021, the Oregon Department of Agriculture treated over 12,000 properties (in an area over 4,000 acres) with Acelepryn G in Washington and Multnomah counties. The project was planned to continue in 2022. More information
Spotted lanternfly — Lycorma delicatula
Spotted lanternfly (Hemiptera: Fulgoridae) is an invasive pest native to Asia that was first detected in Pennsylvania in 2014. It has since spread to multiple counties across Pennsylvania and has been detected in at least 14 states. Spotted lanternflies are easily spread. They lay their eggs on many different surfaces and man-made objects including vehicles. Spotted lanternflies can cause direct damage to plants by feeding on sap. They cause secondary damage by secreting sticky honeydew, increasing the chance of mildews and molds. They have a wide host range and pose a threat to food crops and ornamental nursery crops. In 2020, two detections of dead spotted lanternflies were reported to Oregon Department of Agriculture. These individuals arrived in shipments of packaged goods. More information
Box tree moth — Cydalima perspectalis
Box tree moth is a species of concern detected in Toronto, Canada, in 2018. Box tree moths are native to eastern Asia, but were detected in Germany in 2006 and continued to spread across Europe, where they became a serious economic pest of ornamental nurseries and landscapes. They have not been detected in the United States as of this publication.
The young larvae of box tree moths feed on the undersides of foliage of boxwood plants, making it appear that the leaves are “peeling.” Older larvae will feed on all leaf tissue except for the midribs. Larvae will also consume the bark of defoliated plants. In high populations, boxwood stands have declined up to 95% in eight years or less following introduction in some areas.
In 2021, USDA APHIS published New Pest Response Guidelines for box tree moth.
Boxwood blight — Calonectria pseudonaviculata
The pathogen that causes boxwood blight was first detected in the United States in 2011. Since then, it has spread to 30 states around the nation. A second, related pathogen, Calonectria henricotiae, also causes boxwood blight, but it has not yet been found in the United States. The most common hosts are boxwood (Buxus) species and cultivars, but occasionally Pachysandra and Sarcococca may also be infected. Typical symptoms include leaf spots or leaf blight, defoliation and small, black, linear stem lesions. Infection in Oregon can be mild and difficult to detect on nursery stock. However, once environmental conditions become favorable (warm and moist), the disease can become severe within a few weeks. Additional resources:
Resources and references
Nursery resources
- Herbicide Resistance Action Committee
- Insecticide Resistance Action Committee
- IR-4 Project Environmental Horticulture Program
- Oregon IR-4 Program
- Oregon Association of Nurseries
- Oregon Department of Agriculture Nursery and Christmas Tree Program
- Oregon State University Nursery, Greenhouse and Christmas Trees
- Pacific Northwest Pest Management Handbooks
- U.S. Department of Agriculture Agricultural Research Service, Horticultural Crops Research Unit, Corvallis, OR
References
Griesbach, J.A., J.L. Parke, G.A. Chastagner, N.J. Grünwald and J. Aguirre. 2012. Safe Procurement and Production Manual: A Systems Approach for the Production of Healthy Nursery Stock. Wilsonville, OR: Oregon Association of Nurseries.
Hamer, H., administrator. 2019. United States Summary and State Data (Part 51) in 2017 Census of Agriculture. [Online] Washington, DC: USDA National Agricultural Statistics Service. (Accessed Dec. 1, 2021).
Kaur, N., editor. 2021. Pacific Northwest Insect Management Handbook. Corvallis, OR: Oregon State University. (accessed March 31, 2021).
Oregon State University College of Agricultural Science; Department of Horticulture. 2022. Weed Science: PNW weed identification module. Corvallis, OR: Oregon State University. (Accessed March 30, 2022).
Pscheidt, J.W., and C.M. Ocamb, senior editors. 2021. Pacific Northwest Plant Disease Management Handbook. Corvallis, OR: Oregon State University. (Accessed March 21, 2022).
Taylor, A., director. 2022 Oregon Agricultural Statistics and Directory. Salem, OR: Oregon Department of Agriculture.
Acknowledgments
- Thanks to Jay Pscheidt, Jerry Weiland and Marcelo Moretti for their contributions to develop and organize the section on pathogens and weeds.
- Thanks to Chris Benemann for her contribution and summary of the Oregon Department of Agriculture’s Nursery and Christmas Tree Program.
This project was funded by the Northwest Nursery Crop Research Center, USDA-ARS Horticultural Crops Research Center, Corvallis, OR. Additional support provided by USDA-NIFA Crop Protection and Pest Management Extension Implementation Program.
Appendix A. Using PAMS terminology
This system of terminology for IPM was developed for use by U.S. Federal agencies seeking to support adoption of IPM by farmers. The table below summarizes common tactics used in agricultural IPM using a “Prevention, Avoidance, Monitoring, Suppression” classification. We also define (in italics) the ecological purpose that lies behind a particular practice. The PAMS tables throughout the text provide a simple basis for surveying practices that are used at different crop growth stages in terms of their contribution to a comprehensive IPM program.
Prevention
Prevent introduction to the farm
- Pest-free seeds, transplants
Prevent reservoirs on the farm
- Sanitation procedures
- Eliminate alternative hosts.
- Eliminate favorable sites in and off crop.
Prevent pest spread between fields on the farm
- Cleaning equipment between fields
Prevent pests developing within fields on the farm
- Irrigation scheduling to prevent disease development
- Prevent weed reproduction.
- Prevent pest-susceptible perennial crops by avoiding high-risk locations.
Avoidance
Avoid host crops for the pest
- Crop rotation
Avoid pest-susceptible crops
- Choose genetically resistant cultivars.
- Choose cultivars with growth and harvest dates that avoid the pest.
- Place annual crops away from high-risk sites for pest development (even parts of a field).
Avoid crop being the most attractive host
- Trap cropping
- Use of pheromones
- Use crop nutrition to promote rapid crop development.
Avoid making the crop excessively nutritious
- Use nutrition to promote rapid crop development.
- Avoid excessive nutrients that benefit the pest.
Avoid practices that increase the potential for pest losses
- Narrow row spacing
- Optimized in-row plant populations
- No-till or strip till
Monitoring
Collect pests
- Scouting and survey approaches
- Traps
Identify pests
- Use of identification guides, diagnostic tools and diagnostic laboratories
Identify periods or locations of high pest risk
- Use weather-based pest-development and risk models.
- Use soil and plant nutrient testing.
Determine status and trends in pest risks and classify pest severity
- Maintain pest records over time for each field.
Minimize pest risks over time
- Plan an appropriate PAMS IPM strategy, based upon pest status and trends.
Determine interventions based upon risks and economics
- Use of decision-support tools, economic thresholds
Suppression
Cultural
Outcompete the pest with other plants
- Cover crops
Suppress pest growth
- Mulches
Suppress pest with chemicals from crops or other plantings
- Bio-fumigant crops physical
Physical
Physically injure pest or disrupt pest growth
- Cultivation
- Mowing
- Flaming
- Temperature management
- Exclusion devices
Physically remove pests
- Mass trapping
- Hand weeding
Biological
Suppress pest reproduction
- Pheromones
Increase pest mortality from predators, parasites, and pathogens
- Conservation biological control
- Inundative release and classical biological control
- Use of pest antagonists
Chemical
Use least-risk, highest-efficacy pesticides
- Use economic thresholds to determine that pesticide use is economically justified
- Use pesticides as a last resort, as part of a PAMS IPM strategy
Appendix B. Pest profiles
Information and descriptions in this section are from the Pacific Northwest Insect Management Handbook and the Oregon State University College of Agricultural Science Nursery, Greenhouse and Christmas Tree Program.
Invertebrates
Ambrosia beetles, bark beetles, shothole borers, multiple species
- Shothole borer, Scolytus rugulosus
- European shothole borer/pear blight beetle, Xyleborus dispar
- Lesser shothole borer, Anisandrus saxeseni
Note: Other species may be pests depending on hosts.
Shothole borers, also known as ambrosia beetles, are small bark beetles that were introduced to North America and have been found in the Pacific Northwest since the early 1900s. Apple, pear, cherry and plum are potential hosts. Borers are primarily a problem on injured or stressed plants, but healthy trees growing adjacent to blocks of neglected trees also may be attacked. Shothole borers often attack weakened branches. Shothole borers overwinter as larvae burrowed beneath the bark of infested trees. They pupate beneath the bark. Adults emerge in spring or early summer, mate and fly to susceptible trees to feed at the base of leaves or small twigs. The adults then tunnel into the tree, excavating galleries parallel to the wood grain. They lay eggs along the gallery. The eggs hatch, and the larvae feed by tunneling at right angles to the main burrow, causing a characteristic pattern of damage. After six to eight weeks, the larvae pupate at the ends of the galleries, then emerge as adults starting in August. The many small, round exit holes this creates gives a "shothole" effect. There are two generations per year.
Aphids, family Aphididae, multiple species
- Green peach aphid, Myzus persicae
- Cabbage aphid, Brevicoryne brassicae
- Melon aphid, cotton aphid, Aphis gossypii
- Spirea aphid, citrus aphid, Aphis citricola
- Foxglove aphid, Aulacorthum solani
- Wooly aphids
- Wooly apple aphid, Eriosoma lanigerum
- Wooly ash aphid, wooly elm aphid, Eriosoma americanum
- Wooly beech aphid, Phyllaphis fagi
Aphids are small, soft-bodied insects with piercing-sucking mouthparts formed into a long, needle-like stylet they hold under their body when not feeding. They are usually identified by host plant and characteristics such as color, wing venation, antennae and cornicles (two tubular structures on the hind section of the aphid). The same species can vary in color, shape and size depending on the time of the year. Immature nymphs look like smaller versions of adult nymphs. Some aphid species produce waxy strands over their surface. Crop damage includes distorted plant growth — particularly terminals; deposition of a sweet, shiny liquid called honeydew; black sooty mold (which grows on the honeydew); waxy deposits (some species); yellowing; plant galls (on some hosts); and general plant decline. Several aphid species are also vectors of plant diseases, particularly viruses. Identification of problematic species should be the first step in a management plan. Consult the Pacific Northwest Handbook for your crop to find information about likely problematic aphid species.
Wooly aphids are a group of aphids that can become a serious pest. Adult wooly aphids are reddish to often completely covered with a thick, white, cotton-like substance secreted from the aphid’s abdomen. The insects feed on roots, trunks, limbs and shoots, producing galls at the site of the infestation. Heavy infestations on roots or above-ground portions of the tree can stunt growth and even kill young trees.
Azalea lace bugs, Stephanitis pyrioides
A new introduction to the Pacific Northwest. Little is known about the phenology of azalea lace bug in this region. Azalea lace bug eggs first hatch in early summer, with two to three generations in the Pacific Northwest likely. Nymphs, the young immature lace bugs, are nearly translucent and light yellowish-green when small. As they age, they darken and become spiny. The adult nymphs are around one-quarter-inch long, with wings that are slightly colored with white-and-black patterns in a windowpane effect and quite sculptured. The head capsule is round and swollen-looking from the side.
Lace bugs have piercing-sucking mouthparts. The initial damage shows up as light yellow stippling on the surface of the leaves. Higher populations can cause more severe damage on azaleas, causing the leaves to turn nearly white. On rhododendrons, severe damage may look like iron chlorosis with yellow leaves and green veins. Lace bugs leave small black fecal spots on the underside of leaves. Exuvia, or cast skins, are also often present.
Cucumber beetles
- Spotted cucumber beetle, Diabrotica undecimpunctata
- Striped cucumber beetles, Acalymma trivittata
The adult western spotted cucumber beetle is yellowish green, one-quarter-inch long and has 11 black spots on its wing covers. A close relative, the western striped cucumber beetle, is yellowish and has three black lines down its back. Mature larvae are white, except for the head and last abdominal segment, which are brown, giving the appearance that larvae have two "heads." The larvae are 0.63-inch long. Cucumber beetle adults eat small holes in the leaves and flowers of many crops. The larvae live in the soil where they feed on roots and bore into the base of stems.
Cutworms, Lepidoptera, multiple species
- Variegated cutworm, Peridroma saucia
- Yellowstriped armyworm, Spodoptera ornithogalli
- Winter cutworm, Noctua pronuba
There are multiple moth species with larvae (cutworms) that have a wide range of colors, markings and patterns. The variegated cutworm is commonly found in gardens in the Pacific Northwest. The larvae are black with brown and white markings and measure one-half- to three-quarter-inch long. Damage includes leaf and shoot feeding and may include cutting plants off at the soil line. Cutworm larvae are nocturnal, and their damage can be easily confused with slugs. But cutworms make clean cuts, while slugs rasp from the side of the plant, leaving a ragged edge. Cutworms leave pellet-shaped droppings, while slugs deposit s-shaped sludge wrapped in slime.
Cutworms are found throughout the year, but are most damaging early in the spring when overwintering larvae feed on emerging plant tissues. In the Pacific Northwest, small cutworms feed at night in mid-December and January. Larvae, or the shiny red-brown, bullet-shaped pupa case, also may be unearthed while weeding in spring and early summer. Eggs are laid in patches on plants or nearby grasses
Flea beetles, multiple species
Flea beetle adults are metallic greenish brown to black in color and from 0.06- to 0.12-inch long. They derive their name from their well-developed hind legs; when disturbed, they jump like fleas. The larvae live in the soil, are slender, whitish and about one-quarter-inch long when mature. Adult beetles chew small holes in leaves, giving them a sieve-like appearance. The cotyledons of emerging seedlings are especially susceptible to damage. Larvae feed on underground parts of the plant. High populations of flea beetles feeding on seedling plants can result in stand loss. Foliar damage to mature plants is not considered to be damaging economically. Flea beetles contribute to the spread of various plant diseases.
Fungus gnats, Diptera, Bradysia spp. and Orfelia spp.
Larvae are small white, legless maggots with black head capsules. Adult midges are delicate, black-bodied insects with long legs and antennae. Their wings have a y-shaped vein along the edge, distinguishing them from shore flies, which have five white spots on their dark wings and are stout-bodied with short antennae. The maggots can feed on decaying organic matter as well as plant roots. Larval feeding causes damage to seedling roots, and high populations can kill young plants. Both larvae and adults can move spores of plant pathogens.
Honeylocust pod gall midge, Dasineura gleditchiae
The honeylocust pod gall midge is a tiny orange gnat. The small, bright pink maggots feed within podlike leaves and galls that are deformed and thickened. The larvae are sheltered inside the deformed leaves. Infested leaves may dry and drop from the tree. Small shoots are killed. Although trees are unlikely to be killed, the ornamental quality of the tree may be lost. Thornless varieties of honeylocust are especially subject to damage. Damage from this gall maker is most noticeable in nurseries, but less objectionable in landscapes, especially if the tree is viewed from a distance.
Leafhoppers, Hemiptera, multiple species
Leafhoppers are insects primarily in the family Cicadellidae and the group Cicadomorpha. Several species of leafhopper may attack ornamental plants. Leafhoppers are slender, delicate insects about 0.125 inch or less in length. They are distinguished by the adult hopping or flying to escape danger, and by the ability of nymphs and adults to run forwards, backwards or sideways easily. Leafhoppers have piercing-sucking mouthparts that they use to suck the contents out of surface plant cells. Leafhopper feeding damage to leaves includes small white to yellow stipples, yellowing or leaf curling. Small amounts of this stippling are not injurious to the plants, although sometimes the tips of host plant leaves die and turn brown. Leafhoppers often exude copious amounts of honeydew. Commonly attacked ornamental plants include aster, calendula, gladiolus, dahlia, hollyhock, marigold, rhododendron, rose and zinnia. Some leafhoppers transmit virus diseases to susceptible crop plants.
Leafrollers and leaftiers, Lepidoptera, multiple species
There are many species of moth larvae, including leafrollers and leaftiers, that roll, tie or fold together leaves of ornamental trees, shrubs and other perennials. Leafrollers, when found on native species such as willow, cottonwood, poplar and alder, rarely warrant control since damage is usually cosmetic and short-lived. The leafrolling pests can be divided into single-generation moths, such as the fruit tree leafroller and the European leafroller, and two-generation moths, such as the oblique-banded leafroller and three-lined leafroller. The larvae are mostly green or brown caterpillars with a light brown to black head. Adults are rarely encountered, but have distinctive bands or mottling on the wings. Some are noted for their violent backward wriggling — a means of escape. Newly hatched larvae also may work into blossoms and damage developing fruit, which then abort and fall off the plant. The larvae web the leaves and flowers together beginning in late April, and then feed on the developing fruit or flowers. Feeding on the growing points of young plants can promote undesirable branching. Larvae also feed on the surface of ornamental fruit or berries. Leaftiers are similar in appearance, although larvae are up to one-half-inch long and dirty white, with a brownish head.
Lygus bug, Lygus spp.
The lygus bug adult is about one-quarter-inch long and about half as wide. It is generally brownish with a prominent, V-shaped yellowish area near the center of the body, at the base of the wings. Eggs are bean-shaped, with the outer end blunt or squarely cut off. They are very difficult to spot. Nymphs are yellow-green at first but darken rapidly. They have four dots, often black, on the thorax, and one on the abdominal base. Antennae of the young are generally reddish. Lygus bugs cause different types of damage to different stages and different crops. Lygus bugs are a pest of many agricultural products.
Mites
Two-spotted spider mite, Tetranychus urticae
Several species of spider mites can cause damage in deciduous ornamentals. The appearance of these mites varies with the species, although all are 0.02 inch or smaller. Adults and nymphs can be yellowish, greenish or reddish-brown, depending on species. Mites damage plants by feeding on leaves, which causes stippling, bronzing and possibly leaf drop. The reduction in photosynthesis causes loss of vigor.
Cyclamen mite, Phytonemus pallidus, and broad mite, Polyphagotarsonemus latus
Broad mites and cyclamen mites are extremely tiny and only visible with high magnification. When mature, they measure only about 0.001 inch. Mature cyclamen mites are pinkish-orange and shiny. The hind legs are threadlike in the female and grasping or pincer-like in the male. At low population densities, cyclamen mites are found along the midvein of young unfolded leaves and under the calyx of newly emerged flower buds. At high population densities, these mites can be found anywhere on nonexpanded plant tissue. The mites infest growing tips, young leaves and blooms and cause distorted, twisted and blistered growth. They are spread by wind and by movement of infested stock.
More information: University of California Statewide Integrated Pest Management Program
Nematodes, multiple species
Nematodes, nemas, eelworms and thread or roundworms are synonymous for the same group of organisms. Nematodes usually are regarded as being of microscopic size, though a few species can be seen without magnification. Most plant-parasitic nematodes range in size from 0.02 to 0.04 inch in length. As the common name eelworm implies, a great many nematodes have a wormlike or eellike shape. Females of some kinds grow swollen at maturity and resemble tiny beans, lemons or pears. Nematodes that affect plant growth, called plant-parasitic nematodes, are classified by feeding behavior.
Pacific flathead borer, Chrysobothris mali
The Pacific flatheaded borer is a pest of many different trees and shrubs, including most fruit trees. Adults are reddish-bronze beetles with copper-colored spots on wing covers, about one-quarter- to one-half-inch long. Larvae are whitish to pale yellow and about one-half-inch long when fully developed. Just behind the head of the larvae is a broad, flat enlargement giving a "flat-headed" appearance. Larvae feed beneath the bark and may girdle the trunks and branches of trees. Young and recently planted trees are most susceptible. Trees that are stressed because of drought or other causes are also vulnerable.
Peach tree borer, Synanthedon exitiosa
Peach tree borer is native to North America and common in the Pacific Northwest. The adult is a metallic blue-black, clearwing moth. The male moth may have bands of light yellow scales on the abdomen and resemble a wasp. The female has an orange band around the abdomen. Full-grown larvae are 1 inch long and whitish with a brown head. The larvae burrow into the bark of the crown and feed on the cambium. Feeding and tunnels are restricted to an area a few inches above and below the soil line. Peachtree borer feeding damage can completely girdle and kill young trees. While older trees are rarely girdled, the damage reduces vigor and makes them vulnerable to other pests and diseases. Infested trees "bleed" a reddish amber frass and gum mixture during the growing season.
Root weevils: Black vine weevil, Otiorhynchus sulcatus, and other species
Adult weevils are small, dark beetles with a snout (rostrum) and elbowed antennae. They cannot fly, so distribution is through migration or movement of infested plants, soil or debris. The adults of most of the species are all females and capable of laying eggs. Frequently, adults cause the most conspicuous damage. Adult weevils are night feeders that mostly remain in the soil or in debris at the base of the plant during the day, then climb up to feed on leaves at night. Look for ragged notches on the edges of leaves or flower petals. Larvae, found around roots, are C-shaped, legless and white, or slightly reddish, with tan heads, up to one-half-inch in size. All species are quite similar in appearance and habits of feeding on root hairs, larger roots and root crown. Check the base of unthrifty shrubs for symptoms of girdling by larvae. The various root weevil species differ in susceptibility to pesticides and have different life cycles.
Slugs and snails (multiple species)
- Grey field slug, Deroceras reticulatum
- Multiple Arion spp. slugs including Arion hortensis and Arion rufus
- Striped greenhouse slug, Ambigolimax valentianus
- Tramp slug, Deroceras invadens
- Marsh slug, Deroceras laeve
- Greenhouse slug, Milax gagates
- Brown garden snail, Cornu aspersum
- Amber snails (Family Succineidae)
Slugs and snails are among the most common and persistent pests of home gardens and commercial crops in western Oregon and Washington, and if left unmanaged can cause significant damage. Slugs are closely related to snails, but slugs have no external shell. By day, slugs usually rest in crevices and cracks in the soil, or under surface debris where it is moist. They tend to be active primarily at night, but also feed and reproduce by day during light rain events, foggy periods or after irrigating. Slug damage can be distinguished from that of cutworms, armyworms and other chewing pests by the presence of slime trails and their small, sausage-shaped feces, which are found on or around damaged plants.
More information: Oregon Department of Agriculture Guide to Slugs and Snails in Oregon
Symphylans, Scutigerella immaculata
Garden symphylans are small, white, centipede-like soil arthropods that infest many home gardens and agricultural soils in western Oregon and Washington. They feed on sprouting seeds, roots and other organic material such as decaying plants and fungi. Economic damage occurs from direct feeding on roots, rhizomes and tubers from establishment through plant maturity. Seedling death, poor growth, stunted plants, reduced vigor and yield reduction result. Chronic feeding on the roots of both annual and perennial plants reduces a plant’s ability to acquire water and nutrients. This results in a poor root system that manifests as general stunting and distortion of plants as well as increased susceptibility to plant pathogens. Sampling and control of garden symphylans is complicated by daily and seasonal vertical movement in the soil. Movement is influenced by soil moisture, temperature, time of day, season, crop stage and their internally originating feeding cycles.
Thrips
Western flower thrips (Frankliniella occidentalis) and other species
Several species of thrips cause injury to a number of woody ornamentals. Thrips are tiny, skinny insects, less than 0.05 inch long. Color varies from reddish-yellow to mid-dark brown. Winter populations are darker in color. Thrips have rasping mouthparts and damage plants by sucking out the contents of plant cells and depositing tiny specks of honeydew. Damaged leaves look bleached and silvered and speckled with shiny "tar-spot" excreted by thrips during feeding. They can damage flower buds, opened flowers, leaf buds and leaves. Damage to flowers appears as streaking in the blossoms. There may be yellow pollen on petals where thrips were feeding on the stamens. Western flower thrips are a vector of impatiens necrotic spot virus (see virus profile below).
Whitefly, family Aleyrodidae, multiple species
- Greenhouse whitefly, Trialeuroides vaporarium
- Silverleaf whitefly, Bemisia argentifolia
- Sweet potato whitefly, Bemisia tabaci
Whitefly outbreaks are often associated with warm temperatures and susceptible plant material. The small, football-shaped eggs are laid on the leaf underside with white wax deposits. Small, clear-to-beige, flat scales are evidence of immature whiteflies. The adult whitefly are tiny, yellow-bodied insects with white wings that fly when disturbed.
Weeds
Annual bluegrass, Poa annua (including resistant biotypes)
Annual bluegrass is commonly found in many agricultural areas including gardens, landscape areas, nursery field plantings, vegetable crops, vineyards and orchards. It will establish in almost any disturbed soil area. It can also become quite problematic in golf courses and other turf areas. It has become resistant to several herbicides in the Pacific Northwest.
Bindweed, field bindweed, Convolvulus arvensis
Flowers are trumpet shaped, pink to white in color, with five fused petals. Field bindweed has two leaf bracts that grow from ½ to 1 inch below the flower — this is a key identification characteristic. Flowering is indeterminate, so flowers will continue to develop along growing stems until first frost. Field bindweed grows prostrate until coming in contact with other plants or structures. It is often found growing up and over shrubs and other plants in its path. Field bindweed can be found flourishing in dry, gravelly field soils. It prefers rich, fertile soils with moderate moisture but can tolerate long periods of drought. In moist, warm growing conditions, the leaves are much larger and vines more robust than when drought stressed. Because of its versatility, it is found in many common agricultural plantings, roadsides, railways and pastures.
Bittercress, Cardamine oligosperma and similar species
Plants form a small mounded clump generally 4 to 8 inches tall and wide. However, during warm summer months, bittercress generally grow much smaller. Often, many seedlings germinate in a small area so that they appear as a large, dense mat. Bittercress thrives in moist environments, such as propagation benches and flats, greenhouse floors, gravel container areas, landscape areas and any areas that receive consistent moisture. Bittercress will germinate and grow throughout the year due to the cool environment provided by daily irrigation in container crops.
Canada thistle, Cirsium arvense
Characteristics of Canada thistle are extremely variable when examining populations from different regions. Within the Willamette Valley of Oregon, most plants are similar, ranging from 3 to 5 feet tall. They have glossy foliage on the upper surface and wooly on the lower leaf surface. Leaves are mostly 2 to 8 inches long, alternately arranged, lobed and armed with stiff spines. Canada thistle can be found in cultivated fields, crop fields, riparian areas, pastures, rangeland, Christmas tree plantings, roadsides and other open, disturbed sites.
Common chickweed, Stellaria media
Common chickweed is mostly prostrate and often grows erect by growing on top of itself, forming small mounds or mats. Foliage is opposite, simple and ovate to elliptical. Foliage is generally glabrous (without hairs), although the petioles are hairy, and sometimes the base of the leaf is as well. Common chickweed can grow in a variety of locations. It prefers fertile, moist disturbed sites such as ornamental plantings, lawn areas, gardens and field nurseries. It also thrives in many agricultural crops, vineyards and orchards.
Creeping woodsorrel, Oxalis corniculata
Creeping woodsorrel is a low-growing, spreading plant. The plant spreads by aboveground horizontal stems called stolons. Mature plants form small, mounded clumps commonly 4–8 inches high. Foliage is trifoliate, with three obcordate (heart-shaped) leaflets. The foliage is pubescent, especially on the margins, and usually green to reddish purple in color. Creeping woodsorrel is widely adaptable and commonly grows in yards, landscape areas, agricultural field areas, greenhouses, nursery grounds and containers. Warm, moist environments, provided by greenhouses and common throughout the spring and summer months in Oregon, are ideal environments for creeping red sorrel to germinate and thrive.
Chamomile, dog fennel, Anthemis cotula
Dog fennel is a weed that grows in wet, poorly drained areas. Plants are spindly stalks with few small, dark multi-lobed leaves. Flowers are yellow with white petals. It grows easily in disturbed areas, roadsides, urban and agricultural areas. It has become a problematic weed for agricultural production in the Pacific Northwest in recent decades.
Groundsel, Senecio vulgaris (including resistant biotypes)
Plants are upright, branched and can easily grow 18–24 inches tall. Young plants remain rosettes until reaching maturity. Foliage is green, 2 to 4 inches in length, lacerate (irregularly lobed) and serrate. Groundsel can be found growing just about anywhere — along roadsides, landscape areas, gardens and in most agricultural plantings. Areas of cool, moist, nutrient-rich, cultivated soils encourage rapid growth and reproduction. Three to four generations can develop and thrive in these ideal conditions, causing management issues for growers. Common groundsel typically dies out in non-irrigated areas, or during long, dry, hot periods.
Horseweed, Conyza canadensis
Horseweed (also known as mare’s tail) grows in areas of disturbed soil such as orchards, field nurseries, agronomic crops and unmanaged areas such as roadsides and ditches. Horseweed forms a small rosette in early late winter or early spring. Plants develop slowly in early spring. From the rosette, plants grow almost completely vertical. Horseweed often grows as a single vertical shoot up to 7 feet tall, with a large panicle of flowers on top. Sometimes the plant will branch low near the soil, but it rarely branches above. Leaves are dark green, hairy and without petioles.
Italian ryegrass, Lolium multiflorum (including resistant biotypes)
Italian ryegrass (also known as annual ryegrass) is a common weed in the western United States. It is able to hybridize with perennial ryegrass varieties and is sometimes grown as a cover crop or for forage. It grows easily in many areas, such as orchards, vineyards and crop fields.
Lambsquarters, Chenopodium album
Lambsquarters, also known as goosefoot, is a problematic broadleaf weed in many agricultural systems throughout the summer in Oregon, including nursery and vegetable crops. It grows easily in many agricultural, urban and disturbed areas and can be a source of pathogens that are problematic for some cropping systems.
Liverwort, Marchantia spp.
Liverwort has a flat, dense, branching form. The thallus forms a fleshy mat that grows prostrate over the surface of container crops and greenhouse and nursery floors. Liverworts grow vigorously in conditions with high humidity, high nutrient levels (especially nitrogen and phosphorus) and high soil moisture. Environments that foster any of these three conditions will make control of liverworts difficult. Nursery areas that are moist and humid or any areas that receive consistent moisture such as propagation benches and flats, greenhouse floors and gravel container areas can be especially prone to liverwort infestations.
Northern willow herb, Epilobium spp.
Northern willowherb grows upright, commonly up to 4 to 6 feet tall. Stems are solitary or in clusters, and are rarely branched above. Each leaf is 2 to 8 inches long, serrated, oppositely arranged on lower foliage, but sometimes alternately arranged on the upper part of the stem. Leaves are sessile or with very short petioles. It grows easily in container fields.
Nostoc (Cyanobacteria)
Nostoc is not a plant, but a cyanobacteria that form slick colonies. Nostoc colonies are composed of chains of bacteria that grow on the surface of soil, gravel and even cement. When dry, Nostoc is barely noticeable, forming dark, flaky, paperlike sheets. When wet, it swells up and forms conspicuous green to greenish-brown globby, squishy mats that may carpet alleyways and yards in container nurseries, especially if the ground is compacted and poorly drained. The colonies are covered by a jelly-like sheath that contributes to the colony’s ability to tolerate extended periods of drought and freezing temperatures. These characteristics have also made Nostoc challenging to control in a nursery setting, where it has become widespread on soil, gravel and ground cloth. It is especially challenging for nurseries that grow plants in containers.
Pearlwort, Sagina procumbens
Pearlwort is a low-growing plant that tends to grow upwards, forming small mounds, particularly in nursery containers. It is commonly seen growing as a prostrate mat in containers and on gravel around containers. It grows easily in container fields and greenhouses.
Pigweed, Amaranthus spp.
Redroot pigweed is named for the red, thick taproot it develops. Often, lower stems are also reddish in color. Redroot pigweed has a tall, usually erect habit. It commonly grows 2 to 4 feet tall. With little other vegetative competition, it can reach heights much greater. It develops lateral shoots that allow it to form tall clumps. If mowed repeatedly, this weed can grow and appear prostrate in habit. Mature plants have coarse, hairy stems. It grows easily in container fields, open fields and greenhouses.
Prostrate spurge, spotted spurge, Euphorbia maculata (Chamaesyce maculata)
Prostrate spurge is, as the name indicates, a low-growing plant, rarely growing higher than an inch. Branching often at the base, it forms a mat commonly 6–18 inches across. Prostrate spurge thrives in moist, warm environments, such as propagation benches and flats, greenhouse floors, gravel container areas, landscape areas, and any areas that receive consistent moisture.
Quackgrass, Elymus repens (Elytrigia repens, Agropyron repens)
Quackgrass is a B-listed noxious weed in Oregon. It is problematic for many field-grown crops. It is an invasive perennial grass that can outcompete other grasses and plants. It often grows in clumps, with stalks up to 4 feet tall. It can host several cereal grain diseases.
Shepherd’s-purse, Capsella bursa-pastoris
Shepherd’s-purse is a common broadleaf weed in many agricultural systems, urban areas, roadsides, fields and disturbed lands in temperate areas of the world. It can be an annual or biennial weed. It can be recognized by the small, flat heart-shaped fruits on the mature plants.
More information: Invasive Species Compendium Shepherd's-purse profile
Smartweed, knotweed, Polygonum spp.
Smartweed or knotweeds are broadleaf annuals in the family Polygonaceae. They are sometimes associated with crops or disturbed areas that are near water. There are multiple species of smartweeds or knotweed that are problematic in agricultural systems.
There are also multiple problematic woody knotweed species in Oregon. For more information, see Biology and Management of Knotweeds in Oregon: A Guide for Gardeners and Small-Acreage Landowners, EM 9031.
Wild carrot, Daucus carota
Wild carrot is commonly found in areas of sandy or gravelly soils. It prefers relatively undisturbed sites such as pastures, meadows and Christmas tree plantings; however, it will take hold in cultivated crops if seeds sources are present. It can also be problematic in noncrop areas such as ditches, roadsides and field edges. Wild carrot has small white flowers, each with five petals. Flowers are held in clusters called umbels, and each umbel contains many individual flowers. Often there are one or more purple-tinged flowers in the center of the 3- to 5-inch umbel. Flat-shaped umbels form at the terminal end of flower stalks. Upon ripening of seeds, umbels often close up.
Yellow nutsedge, Cyperus esculentus
Yellow nutsedge grows upright to form dense clumps. Its habit resembles that of many grasses. Its thick, stiff leaves are arranged in sets of three at their bases. A cross section of their stems shows a solid, triangular shape, in contrast to grass stems which are hollow and round, and in cross section are almost flat or oval shaped. Yellow nutsedge is predominantly found in cultivated, irrigated agricultural field sites, such as field nurseries and row crops. It can be found in landscapes and turf areas as well. Yellow nutsedge prefers areas with full sun and plentiful moisture. It does not do well in shaded areas. Once established, yellow nutsedge is extremely difficult to control and is more tolerant of drier conditions.
Plant pathogens
Fungal pathogens
Anthracnose (general grouping of several fungal pathogens)
Anthracnose is a term used to describe many different fungal pathogens. Species that cause anthracnose differ based on the host. For information on different anthracnose-related fungal pathogens, consult the Pacific Northwest handbook for anthracnose associated with your host plant.
Monilinia brown rot blossom blight and fruit rot
The fungi Monilinia fructicola and M. laxa can incite a blossom blight, a twig and branch dieback, and a fruit rot of several Prunus spp., including many ornamental and fruit trees. Fungi survive year to year on infected twigs, branches, old flower parts or mummified fruit. Fruiting and ornamental cherries, peaches, nectarines, prunes, plums, almonds and apricots are susceptible. Pome fruit, including quince, can be susceptible under high disease pressure years. The disease is more of a problem west of the Cascade Range.
Botrytis
Botrytis cinerea (sexual: Botryotinia fuckeliana) and other Botrytis spp. are fungi that colonize dead, dying and wounded plant parts. From these infections they can attack healthy tissues. A moist, humid environment is ideal for pathogen sporulation and spread. Spore dispersal is stimulated by changes in relative humidity. Conidia may come from many sources in and outside the greenhouse. Concentrations peak in the greenhouse during irrigating, spraying pesticides, harvest and shipping. It is found everywhere plants are grown and has a wide host range.
Fusarium
Fusarium spp. are fungal pathogens that can cause a number of symptoms on many crops, including wilts, crown and root rots, blights and damping off.
Powdery mildew
Powdery mildews rank among the most important diseases of food and ornamental plants. Damage can result in death of host tissue (even entire plants), defoliation, cosmetic damage, reduced yields and lowered quality. The unsightly appearance of powdery mildews reduces the economic and aesthetic value of ornamental plants as well as plants that bear fruits and vegetables. Powdery mildews also can cause losses in yield and quality by enabling decay organisms to enter fruits through damaged epidermal tissue. For some crops, such as wine grapes and cherries, controlling powdery mildews determines how the crop is grown (including such practices as pruning and managing soil fertility). Fortunately, effective cultural and chemical control practices are available for nearly every plant species susceptible to powdery mildew.
Bacterial blights
Pseudomonas
Pseudomonas syringae is responsible for a number of economically important diseases in the Pacific Northwest. This bacterium can infect a wide variety of fruits, vegetables and ornamental plants. A variety of symptoms are associated with woody plants infected by Pseudomonas syringae pv. syringae. Symptoms and symptom development depend on the species of plant infected, the plant part infected, the strain of Pseudomonas syringae and the environment. More than one symptom can occur simultaneously on a single plant.
Xanthomonas
Xanthomonas is a genus of bacterium in which multiple species and strains cause a number of symptoms on many crops, including leaf spots, bacterial blights and cankers.
Crown gall
Crown gall continues to be a major problem for the nursery industry, both in woody and herbaceous plants. The pathogen traditionally known to cause crown gall in the most plants is Agrobacterium tumefaciens (Rhizobium radiobacter). The pathogen name has been under dispute for decades, and A. tumefaciens is known to be a species complex, consisting of at least 11 different genomospecies. Crown gall is a tumor-forming disease of plants caused by tumorigenic agrobacteria, many of which are thought to be present in most agricultural soils. The pathogens, in soil or on infested plants, are disseminated by splashing rain, irrigation water, heeling-in galled plants with healthy plants, farm machinery, pruning tools, wind and plant parts used for propagation.
Tip blights
Arborvitae tip blight
The fungus Pestalotiopsis funerea is a common problem on arborvitae. The fungus can colonize tissue damaged from other pathogens, insects, freeze injury or sunscald. Other stresses include being pot bound, too much or too little water or fertilizer. Spores are disseminated from diseased tissue by splashing rain. The fungus has a wide host range of many different conifers and has been associated with leaf and stem blights, cankers, diebacks and even root rots of these hosts.
Spruce tip blight
Sirococcus conigenus (formerly Ascochyta piniperda), a fungus, attacks many conifers including western hemlock; Jeffery, ponderosa and red pine; and blue and sitka spruce. The fungus overwinters in dead shoots. Spores are rain-splashed from one tree or seedling to another. Infection is on or near the base of needles on new shoots as they begin to grow in the spring. Seedling infestation occurs when the fungus colonizes the seed cones. New shoots die back in summer. At first, needles may turn brown starting at the base or fall off in the middle of the shoot near the infection point. If the twig dies during shoot elongation, a characteristic hook shape develops. Diseased twigs slowly die back to the branch where the bud began. Numerous small, black, fruiting bodies (pycnidia) develop on the twig about a year after the infection began. Can be confused with frost or herbicide damage.
Phomopsis
Phomopsis refers to a fungus in which multiple species cause a number of symptoms on many ornamental crops, including tip and twig blights, cankers and root rots.
Leaf spots and blights
Leaf spots
Leaf spots refer to symptoms on many different types of crops that occur due to multiple causal agents. These can be difficult to identify and distinguish from symptoms of other pathogens.
Phytophthora
Although Phytophthora is a recognized disease problem in the Pacific Northwest, it has been often misdiagnosed in Oregon. A wide variety of cultural and chemical controls can be implemented for Phytophthora problems. The first step in managing any of the several diseases caused by Phytophthora spp. is to obtain an accurate diagnosis. Certain lines of evidence can quickly lead you toward (or away from) a Phytophthora disease diagnosis. The evidence is in the field characteristics, field history and symptoms of the affected plants. Knowing the biology of the Phytophthora fungus and conditions that favor its development also helps the diagnosis. Plant samples can also be taken to a laboratory for traditional or “high tech” tests to confirm the presence of this fungus-like organism. See “Sudden oak death,” below.
More information: Preventing Phytophthora Infestations in Restoration Nurseries, EM 9330
Shothole
Shothole symptoms are commonly observed on Prunus spp. and can be caused by a variety of factors. Symptoms develop in response to injuries of mechanical, chemical or pathogenic nature. Meristematic tissue around the injury becomes lignified or suberized to form a physical and biochemical barrier that, in the case of pathogens, limits the colonization of healthy tissue. The formation of an abscission layer may depend on temperature. The bacteria Pseudomonas syringae pv. syringae and Xanthomonas arbicola pv. pruni as well as several fungi including Cercospora sp., Blumeriella sp., and Thyrostroma carpophilum (Coryneum blight) can cause leaf spots and shothole on cherry laurel (English laurel, Otto Luyken or 'Zabeliana'). Thyrostroma carpophilum (formerly Wilsonomyces carpophilus) is observed more frequently than Blumeriella jaapii (formerly Coccomyces prunophorae). Both fungi are favored by warm, wet conditions in spring. Several other fungi and bacteria have been found to cause shothole symptoms. Of particular concern is the oomycete Phytophthora ramorum, which is a regulated organism.
Note: These symptoms are not to be confused with injury caused by shothole borers, beetles that dig into the woody and meristematic tissues of plants.
More information:
- Prunus laurocerasus — leaf spots and shothole
- Cherry, flowering (prunus spp.) shothole (corneum blight)
- Prune and plum — leaf spots
Root and crown rots
Phytophthora
See Phytophthora in leaf spots and blights, above.
Fusarium
Fusarium spp. are fungal pathogens that can cause a number of symptoms on many crops, including wilts, crown and root rots, blights and damping off.
Black root rots — Berkeleyomyces spp. (Thielaviopsis basicola)
Berkeleyomyces sp. (formerly Thielaviopsis basicola) is a widely distributed root pathogen reported from at least 30 plant families, including many ornamental plants. Untreated or improperly treated field soil can be a source of T. basicola if used in potted-plant culture. Most soilless media including commercial peat moss are clean. T. basicola spores from other areas of a greenhouse facility can contaminate potting media and result in disease problems. The pathogen forms thick-walled chlamydospores, which can survive several years in soil.
Wilts
Verticillium wilt
The fungus Verticillium dahliae is the primary causal agent of Verticillium wilt. Nine other species of Verticillium have also been found to cause wilt in certain hosts, but none has as wide of a host range as V. dahliae. The disease affects herbaceous annuals and perennials as well as woody trees and shrubs. Plants affected by Verticillium wilt can exhibit chlorosis, wilting, defoliation and premature senescence. The disease can reduce the yield of agricultural crops as well as the lifespan of plants in landscapes and perennial cropping systems — ultimately resulting in greater inputs, increased costs of production and economic loss.
Pathogens of specific concern
Phytopthora ramorum — Sudden oak death
Sudden oak death is a disease caused by the fungal-like pathogen Phytopthora ramorum. It is known to infect at least 100 different plant species. This pathogen has been detected in multiple counties in Oregon. It is of particular concern because it can quickly kill members of the family Fagaceae, such as tanoaks and coastal live oaks. The pathogen can infect other plant families, including some that are produced in ornamental nurseries. There is concern that the pathogen can be spread quickly throughout the state and region. Because of the high potential for spreading this serious disease, there is currently an program by Oregon Department of Agriculture and USDA APHIS PPQ to stop the spread of this disease.
More information:
- Sudden Oak Death and Phytophthora ramorum: A Guide for Forest Managers, Christmas Tree Growers, and Forest Tree Nursery Operators in Oregon and Washington
- Preventing Phytophthora Infestations in Restoration Nurseries, EM 9330
- SOD Program
Erwinia amylovora — Fire blight
Erwinia amylovora is a bacterium that overwinters in cankers on infected pear, apple and some ornamental trees. This bacterium causes the disease known as fire blight. During moist periods in the bloom season, spurs, blossoms and twigs may become infected, developing a darker, water-soaked appearance. They turn brown to black and rapidly wilt and die. Infected blossoms frequently are distorted. A tan-yellow bacterial exudate, or ooze, often appears where infection occurs. Insects, pruning tools and splashing rains spread this bacterium. Risk of infection increases with the number of more active "holdover" cankers in an orchard.
Viruses
Impatiens necrotic spot virus and tomato spot wilt virus
Impatiens necrotic spot virus and tomato spot wilt virus are two viruses with a wide host range. While intitally thought to be two strains of the TSWV, INSV is a different virus. TSWV and INSV have wide, overlapping host ranges and produce a broad range of symptoms on each plant species. Although TSWV is often found in tuberous dahlias and chrysanthemums, INSV is commonly found on other greenhouse crops such as impatiens, cyclamen and gloxinia. Vegetable transplants such as tomato, eggplant, peppers, lettuce and basil can also get both viruses.
Symptoms of both diseases vary according to host, time of infection and environmental and cultural conditions. TSWV and INSV symptoms can mimic those of fungal, bacterial or other virus infections. Both viruses can produce necrotic (dead) spots on leaves, discolored or black areas that follow leaf veins, general or spotty chlorosis (yellowing), black or purple stem streaks or leaf spots, falling of leaves or buds, leaf distortion, blackening of young leaves and growing points, stunting, and colored spots, stripes, or rings on petals, leaves, or fruit. The ringspot symptom is often seen.
Tobacco mosaic virus or tomato mosaic virus
Tomato mosaic virus, or tobacco mosaic virus, affects many ornamental and edible greenhouse crops. Poor sanitation and handling infected plants are the primary means of spread. Symptoms depend on the virus strain. Often, foliage mottles with alternating yellowish and dark green areas. New leaflets curl and are slightly malformed. More severely affected leaves are badly distorted and rather fern-like. Plants usually are somewhat stunted and bear few fruit if infected when young. Plants and fruit are not much smaller if plants are infected after they reach fruiting stage. Sometimes necrotic spots or streaks develop on stems and leaves.
Use pesticides safely!
- Wear protective clothing and safety devices as recommended on the label. Bathe or shower after each use.
- Read the pesticide label—even if you’ve used the pesticide before. Follow closely the instructions on the label (and any other directions you have).
- Be cautious when you apply pesticides. Know your legal responsibility as a pesticide applicator. You may be liable for injury or damage resulting from pesticide use.